electron geometry of xef4|electron geometry chart : Clark XeF 4 contains 4 bonded and 2 nonbonded electron domains, giving an octahedral e-domain geometry and a square planar molecular geometry. (AX 4 E 2 ). A cartoon .
Samsung's latest HBM chips have yet to pass Nvidia's , opens new tab tests for use in the U.S. firm's AI processors due to heat and power consumption problems, three people briefed on the issues .US Time Zones
PH0 · xef4 lewis structure molecular geometry
PH1 · po4 3 electronic geometry
PH2 · molecular geometry of xef4
PH3 · electron geometry vs molecular geometry
PH4 · electron geometry chart
PH5 · electron and molecular geometry table
PH6 · c2h4 electron pair geometry
PH7 · Iba pa
Mediacorp CAPITAL 958 城市频道 - Videos - Facebook
electron geometry of xef4*******Learn how to draw the Lewis structure of xenon tetrafluoride, a square planar compound with two lone pairs on xenon. Find out the molecular geometry, hybridi.
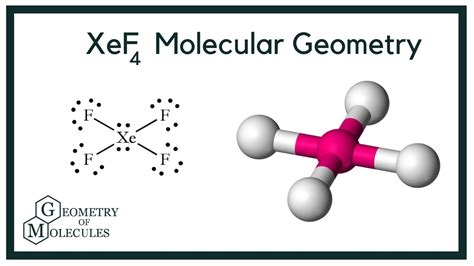
To summarize this blog post, we can say that XeF4 has 36 valence electrons. It has two lone pairs of nonbonding electrons on the central atom of Xenon. The molecule has octahedral electron . To summarize this blog post, we can say that XeF4 has 36 valence electrons. It has two lone pairs of nonbonding electrons on the central atom of Xenon. The molecule has octahedral electron .

Xenon tetrafluoride is a chemical compound with chemical formula XeF 4. It was the first discovered binary compound of a noble gas. It is produced by the chemical .
XeF4 Lewis Structure, Molecular and Electron Geometry based on the VSEPR theory, the steric number, Hybridization and expected bond angles.What is the molecular geometry of XeF4? The molecular shape of XeF 4 is square planar, or AX 4 E 2 using Valence Shell Electron Pair Repulsion (VSEPR) theory. Hence, the .XeF 4 contains 4 bonded and 2 nonbonded electron domains, giving an octahedral e-domain geometry and a square planar molecular geometry. (AX 4 E 2 ). A cartoon .For the XeF4 Lewis structure we first count the valence electrons for the XeF4 molecule using the periodic table. Once we know how many valence electrons there are in XeF4 .Other articles where xenon tetrafluoride is discussed: chemical bonding: Applying VSEPR theory to simple molecules: The XeF4 (xenon tetrafluoride) molecule is hypervalent with six electron pairs around the central xenon (Xe) atom. These pairs adopt an octahedral arrangement. Four of the pairs are bonding pairs, and two are lone pairs. According to .electron geometry of xef4 electron geometry chartThe central atom has 6 electron pairs out of which two are lone pair electrons. The hybridization in Xenon is sp 3 d 2 because there is a migration of two electrons of p to d orbital which results in the formation of sigma bond with F. XeF 4 Molecular Geometry And Bond Angles. XeF 4 consists of two lone pair electrons. Now if we follow the .See Answer. Question: Use VSEPR theory to predict the electron-pair geometry and the molecular geometry of xenon tetrafluoride, XeF4. Select one: a. The electron-pair geometry is octahedral, the molecular .Verified by Toppr. The structure of XeF 4 is shown below. The central Xe atom has 2 lone pairs and 4 bond pairs of electrons. The electron pair geometry is octahedral and molecular geometry is square planar. Xe atom undergoes sp3d2 hybridisation.
The valence electrons refer to the outermost electrons in the atom of a chemical element. Valence shell electron pair repulsion predicts the three-dimensional structure of a compound by explaining the effects of the electron repulsion to the overall geometry of the molecule. Answer and Explanation: 1Figure 7.2.2. (a) The electron-pair geometry for the ammonia molecule is tetrahedral with one lone pair and three single bonds. (b) The trigonal pyramidal molecular structure is determined from the electron-pair geometry. (c) The actual bond angles deviate slightly from the idealized angles, because the lone pair takes up a larger region of .electron geometry chartStep 1. To determine the electron geometry ( eg) and molecular geometry ( mg) of XeF A 2, we need to follow thes. Determine the electron geometry (eg) and molecular geometry (mg) of XeF2. A) eg trigonal bipyramidal, mg-bent B) eg linear, mg linear C) eg-tetrahedral, mg linear D) eg-trigonal bipyramidal, mg linear E) eg-tetrahedral, mg-bert 3. X=Number of surrounding atoms. E= Number of lone pairs on central atom. For this one, we can see that it has one central atom (Xe), four surrounding atoms (F), and two lone pairs of electrons around the central atom, making it AX 4 E 2. Step 3: Use the VSEPR table to determine the geometry of XeF4.
For the XeF4 Lewis structure we first count the valence electrons for the XeF4 molecule using the periodic table. Once we know how many valence electrons there are in XeF4 we can distribute them around the central atom and attempt to fill the outer shells of each atom. The Lewis structure for XeF4 has a total of 36 valence electrons.XeF4 Geometry and Hybridization. Xe is the central atom: There are 4×7 + 8 = 36 electrons and 8 are taken to make 4 covalent bonds. Each fluorine takes 3 lone pairs, so there are 36 – (8+4×6) = 4 electrons left which go to Xe as 2 lone pairs: There are 4 atoms and 2 lone pairs on the central atom, therefore, the steric number is 6 and the . The electron geometry of XeF4 is octahedral, due to its arrangement of two lone pairs and four bonds. However, its molecular geometry is square planar because the lone pairs are directly across from each other. Explanation: The electron geometry (eg) and molecular geometry (mg) of XeF4 can be determined based on its molecular .Predict the electron pair geometry (EPG) and molecular geometry (MG) of the following compounds based on the Lewis structure and VSEPR: A. H2O B. PF5 C. CO2 D. SO2 E. CHCl3 Predict the electron pair geometry and the molecular structure of each of the following: (a) IOF5 (I is the central atom) (b) POCl3 (P is the central atom) (c) Cl2SeO .Covalent molecules or ions are one of the main types of chemical compounds. They all involve one or more valence electron pairs being shared by two or more connecting atoms. Except for simple diatomic molecules or ions, there is usually a central atom present bonded to multiple peripheral atoms. This central atom may also contain non-bonding .Thus, the electron-pair geometry is tetrahedral and the molecular structure is bent with an angle slightly less than 109.5°. In fact, the bond angle is 104.5°. Figure 7.2.7. (a) H2O H 2 O has four regions of electron density around the central atom, so it has a tetrahedral electron-pair geometry. The electron geometry describes the arrangement of electron pairs around the central atom. In XeF4, there are 4 bonding pairs (Xe-F) and 2 lone pairs on Xe. The presence of 6 electron pairs suggests an octahedral electron geometry, where the pairs are arranged in a symmetrical manner around the central atom. 3. Determine the .
Oxygen has six valence electrons and each hydrogen has one valence electron, producing the Lewis electron structure. Figure 10.2.2 10.2. 2: (CC BY-NC-SA; anonymous) 3. With two bonding pairs and two lone pairs, the structure is designated as AX 2 E 2 with a total of four electron pairs.electron geometry of xef4The electron geometry is octahedral, while the molecular geometry is square planar, Xenon has 6 nonding electron pairs, therefore the electron geometry of octahedral, but two of the pairs of electrons on the central atom are unbonded, or lone pairs therefore the molecular geometry is square planar. Was this answer helpful?Draw the Lewis structure for XeF4 in the window below and then answer the questions that follow. b What is the electron-pair geometry for Xe in XeF4 ? c What is the shape (molecular geometry) of XeF4? There are 3 steps to solve this one.
We would like to show you a description here but the site won’t allow us.
electron geometry of xef4|electron geometry chart